Formulate. Comply. Manage.

Formulate. Comply. Manage.
Cosmetri software empowers cosmetics and personal care companies by streamlining operations, accelerating product development and ensuring compliance with ever more stringent regulations. Our industry-leading applications are Product Manager for cosmetics PLM and Cosmetri GMP for cosmetics GMP compliance, including ISO 22716. Cosmetri software is fully scalable and used by small brands up to corporations with an annual turnover up to $20 billion.
What our clients say

What our clients say
Cosmetri allows me to collate all of my formulations, ingredients, and compliance information in one location which can be accessed readily. It has become an indispensable tool in my work. Cosmetri allows me to remain highly organized and ensure that I am meeting compliance requirements.
We have demonstrated to our auditor how raw materials and production dispense lists are managed using Cosmetri and therefore we have successfully achieved the GMP ISO 22716 certification again this year in early 2021.
Cosmetri has revolutionised our workflow. From thirty spreadsheets a day to one slick software application and hours less work. I wish we had found it sooner!
We are a skin care manufacturer and Cosmetri is an invaluable piece of software that is central to our manufacturing processes. The service is fantastic, and queries are dealt with in a timely and helpful manner.
What I love about Cosmetri is that I know for sure we are compliant with the regulations as long as we are using Cosmetri. It keeps my products and business safe.
Cosmetri is without doubt the most comprehensive and user-friendly compliance software for our industry. We used it to obtain and maintain our GMP ISO-22716. From the management of raw materials to final batch protocols the system just delivers.
We have been using Cosmetri software for some time, upgrading to the Enterprise version within one year. The software saves us a lot of time in the business and provides an efficient system for organizing and managing our products. The support is also excellent, fast and friendly!
What I love about Cosmetri is how easy it makes R&D work. I can easily create stability testing programs and manage multiple formulations. It basically does the work of one employee. Having used other similar R&D software, cosmetri is superior to all of them due to its high functionality and ease of managing large amounts of data.
HFB Labs rely on Cosmetri‘s Product Manager for ensuring the compliance of our formulations and management of regulatory data and documents. The software is invaluable for formulating new products with user-friendly tools that enable precise control of ingredients used in different clients’ formulas.
We are using Cosmetri for the last year mainly for regulatory reasons. It made our daily business a lot more efficient. The software is easy to use and covers most of our needs. Support is impeccable, the few times we had to contact them they answered promptly and solved the issues within a very short time. Summarised, a highly recommended software suite if you are working in the cosmetic field!
We started our business three years ago and began using Cosmetri immediately. I was so happy that we found a software that covered all our needs. From formulation planning, PIF generation to safety data analyses and calculations (SEM, MoS etc). Without this powerful tool it would have been impossible to fulfill all the compliance and regulation tasks in reasonable time. Nowadays, we use every function of Cosmetri, and this software is at the heart of our business. Last month we realized our ISO:22716 certification. Cosmetri and all the documentation provided was a real big help.
Join the hundreds of cosmetic companies that trust us to help with formulation and compliance solutions.
Software Features
Product Manager and Cosmetri GMP provide comprehensive software tools for the following:
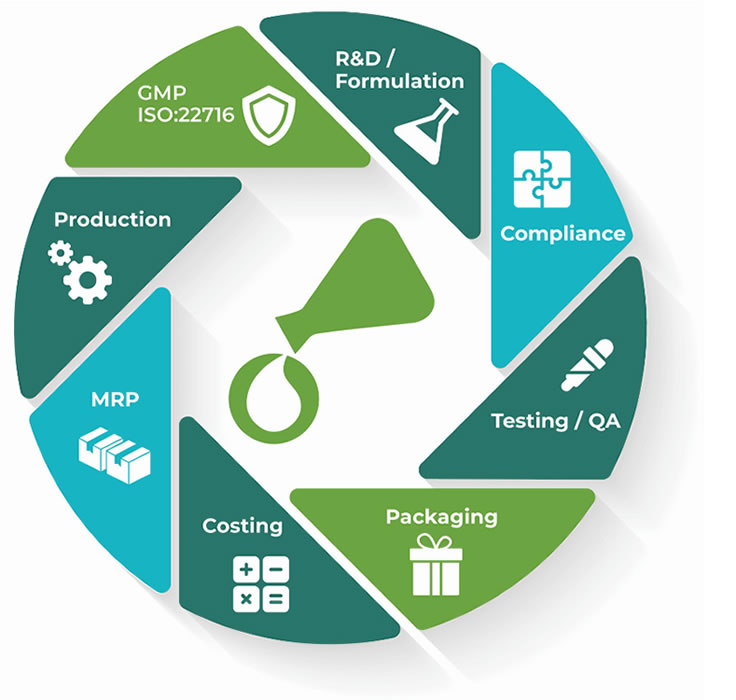
R&D / Formulation

R&D / Formulation
Create projects to clarify the concept, customer demographic and other key data forming the NPD brief. Build project checklists for effective project management. Clone an approved project’s data to any linked products as the basis for the specification. Intuitive, fast formulation with overview of all formula versions and their statuses. Comprehensive formula samples management with QA and inventory control. Advanced formula comparison and reporting features. Select a master formula for a product and lock all associated items.
Compliance
View the formula’s INCI breakdown by %w/w, including calculation of fragrance allergens and additional color pigments. Manage compliance of the formula by compliance zones/countries. Compliance Pack includes regulatory data for 50+ countries. Ingredients Watch warns of changes by an authority to ingredients in your formulas. Build custom advisory lists for control of ingredients that should be included or excluded from the formula. Advanced LOI management and template-based specification reporting.
Compliance

Testing / QA

Testing / QA
Configure test groups for precise quality management and standardization of all workflows. QA checklists ensure adherence to standard operating procedures and full traceability. Fast test configuration and results entry. Conduct stability testing on retained production batches and formula samples. CoA generation, batch protocols and comprehensive test reports. Auto-change approval statuses when a raw material or formula expires or a test is not completed. Lock all approved items,
Packaging
Build packaging sets for each unit size of a product, including assembly instructions. Apply QA to packaging components, allowing only approved batches to be available for pack-out. Fast manual dispense of packaging sets or automated FIFO-based dispense at the pack-out stage of a manufacturing order. Test packaging sets at R&D stage along with formula samples. Full packaging inventory management, including MRP, costs and dispense lists.
Packaging

Costing

Costing
View and optimize the formula’s materials costs per L/kg as you formulate. Enter materials and packaging costs in any currency. Conversion of foreign currency costs using current exchange rates. Enter optional price points/arrays, production and other cost factoring. Calculation of BoM costs for any production amount. Easy calculation of profitability of each unit size, with side-by-side comparison of costs and profit margins for different formula versions.
MRP
Fast scheduling of future production requirements. Comprehensive material requirements planning for raw materials and packaging. Separate or combined MRP reporting for production and/or sample development inventory requirements. Conduct stock checks and export inventory reports. Advanced filtering and reporting of materials’ inventory based on QA status, supplier, properties, expiry date, compliance, etc.
MRP

Production
Fast, user-friendly management of production orders with integrated quality control and testing, including SOP checklists. Separate production workflow for QA formula samples. Enter amounts in either weight or volume. Batch protocols for traceability, including dispense lists, batch tickets and in-process test reports. Generation of production reports, including by customer group. Configure QA to prevent production using non-approved formulas or raw material/packaging item batches.
GMP ISO 22716
Cosmetri GMP application enables compliance with GMP ISO 22716 for cosmetics manufacturers. Works in conjunction with Product Manager or standalone. Includes advanced EDMS for SOP, WI etc document management, SOP template kit and role-based staff management. Features include product recalls, customer complaints, supplier reviews, OOS investigations, internal audits, UE/SUE reporting and CAPA-based change control.
Request a Software Demo
Click below to submit your details. You’ll be assigned a specialist from our team who will guide you through every step and answer any questions.
Discovery Session and Software Trial